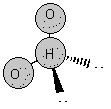
A. Dependent on the 3D structure of the molecule.
1. Molecules are not always flat, 2D objects which we depict on paper.
2. Molecules have a 3 dimensional shape which affects properties like
a.
b.
c. polarity
1) polarity = unequal charge distribution in molecule = partial charges ( d+ ) ( d- )
a) based on presence of polar covalent bonds
b) presence of non-bonding pairs of electrons
c) 3D shape of molecule: presence of asymmetry
2) examples of types of polar molecules:
3) examples of types of non-polar molecules:
4) determining polarity in this class:
a) non-polar molecules:
b) polar molecules
3. Shape/polarity affects how molecules interact with each other
a. intermolecular interactions or cohesive forces
b. how molecules "see" each other: attractive forces between molecules
4. 3D shape is determined by how many sets of electrons radiated out from a central atom.
a. remember, electrons carry a negative charge therefore, covalent bonds and non-bonding pairs of electrons will try to get as far away from each other as possible.
b. 2 covalent bonds present: angle = shape =
c. 3 covalent bonds present: angle = shape =
d. 4 covalent bonds present: angle = shape =
e. what happens to the shape of molecules when there are non-bonding pairs on the central atom?
1) 2 covalent bonds, 1 non-bonding pair: angle = shape =
2) 3 covalent bonds, 1 non-bonding pair: angle = shape =
3) 2 covalent bonds, 2 non-bonding pairs: angle = shape =
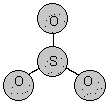
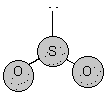
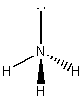
5. types of 3D shapes "observed" with simple molecules
Molecular Structure Bond Angle Example symmetry/polarity linear 180o symmetrical non-polar trigonal planar 120o symmetrical non-polar angular <120o asymetrical polar tetrahedral ~109o 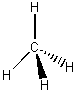
symmetrical non-polar trigonal pyramidal <107o asymmetrical polar angular <104.5o asymmetrical polar
B. Types in intermolecular interactions
1. Hydrogen bonds
a. when do they occur?
b. how strong is the interaction?
c. how is this phenomenon depicted (cartooned?)
d. why does it happen?
2. Dipole interactions or polar interactions
a. when do they occur?
b. how strong is the interaction?
c. how is this phenomenon depicted?
d why does it happen?
3. Hydrophobic interactions (non-polar interactions)
a. when do they occur?
b. how strong is the interaction?
c. why does it happen?
1)
2)
3)
4)
example of size dependent bp:
CH4 = -161oC C2H6= -88oC C3H8= -42.1oC
C4H10 = -0.5oC C5H12= 35-36oC C6H14= 69oC
C7H16= 98oC C8H18= 125-127oC